Nucleic Acid Diagnostic Kit for SARS-CoV-2(Fluorescence PCR)
WHO recommended, standard confifirmation of acute SARS-CoV-2 infections is based on the detection of unique viral sequences by nucleic acid amplifification tests (NAATs), such as real-time reverse-transcription polymerase chain reaction (rRT-PCR) . PCR Assay detects SAR-CoV-2 in the window period and find the infected as early as possible.
Fluorescence PCR, gold standard
Upper respiratory specimens:
Nasopharyngeal (NP) swab / Oropharyngeal (OP) swab
Detects SAR-CoV-2 in the window period and find the infected as early as possible.
3 product categories correspond to 4 different genes detection combinations
*WHO recommend, NAAT(PCR) assay with at least two independent targets on the SARS-CoV-2 genome.
FEATURES
● Specific gene ORF1ab, N gene and E gene.
3 product categories correspond to 4 differentgenes detection combinations
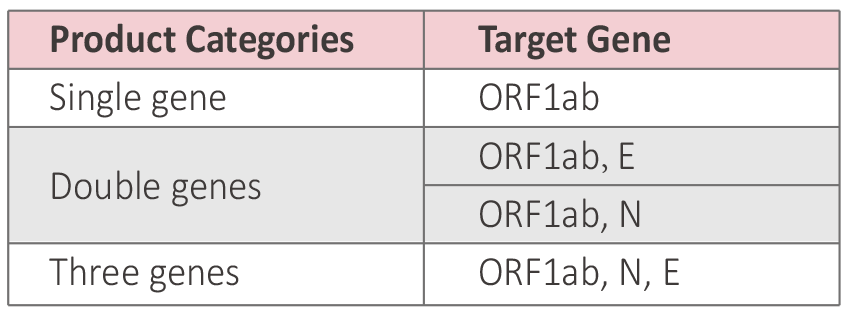
● Specific gene ORF1ab, N gene and E gene.
lncluding negative and positive quality control
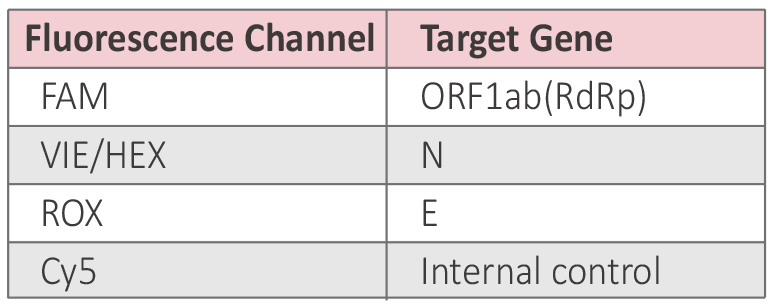
● Multiple Fluorescence Channels
4 channels: FAM, VIC/HEX,ROX,CY5
● Efficient and fast
Based on one-step RT-PCR and Taqman technology
- +852-3500-5196
- info@kindmay.com
-
32nd Floor, Block B, Building 10,
Shenzhen Bay Science & Technology Ecological Park,
Nanshan, Shenzhen 518057 China

 Language
Language



